Fun fact: did you know that the word “literature” appears approximately 30 times in MEDDEV 2.7/1 rev.4?
If you’re involved in the preparation of Clinical Evaluation Reports (CERs), you are likely beginning to realize the important role that literature reviews play in meeting the new EU regulatory requirements that came into effect in May 2021.
As a fundamental component of CERs, literature reviews are used to:
- Identify and evaluate safety and performance data;
- Establish state of the art; and,
- Monitor risks and reconfirm device risk profile.
One important thing to remember: each indication needs its own literature review, which can add up fast!
With regulatory bodies increasing their scrutiny of literature reviews and CERs in general, many organizations are looking for new ways to consistently deliver regulatory compliant, audit-ready literature reviews on time and on budget.
As with many things in life, the key to getting good results is starting with a strong foundation. That’s why our #1 tip for better literature reviews is:
Use the PICO framework to perfect your research question.
The Value of Good PICO Questions
Good literature reviews start with a rock-solid research question. Using the PICO framework will help ensure that your research question is effective.
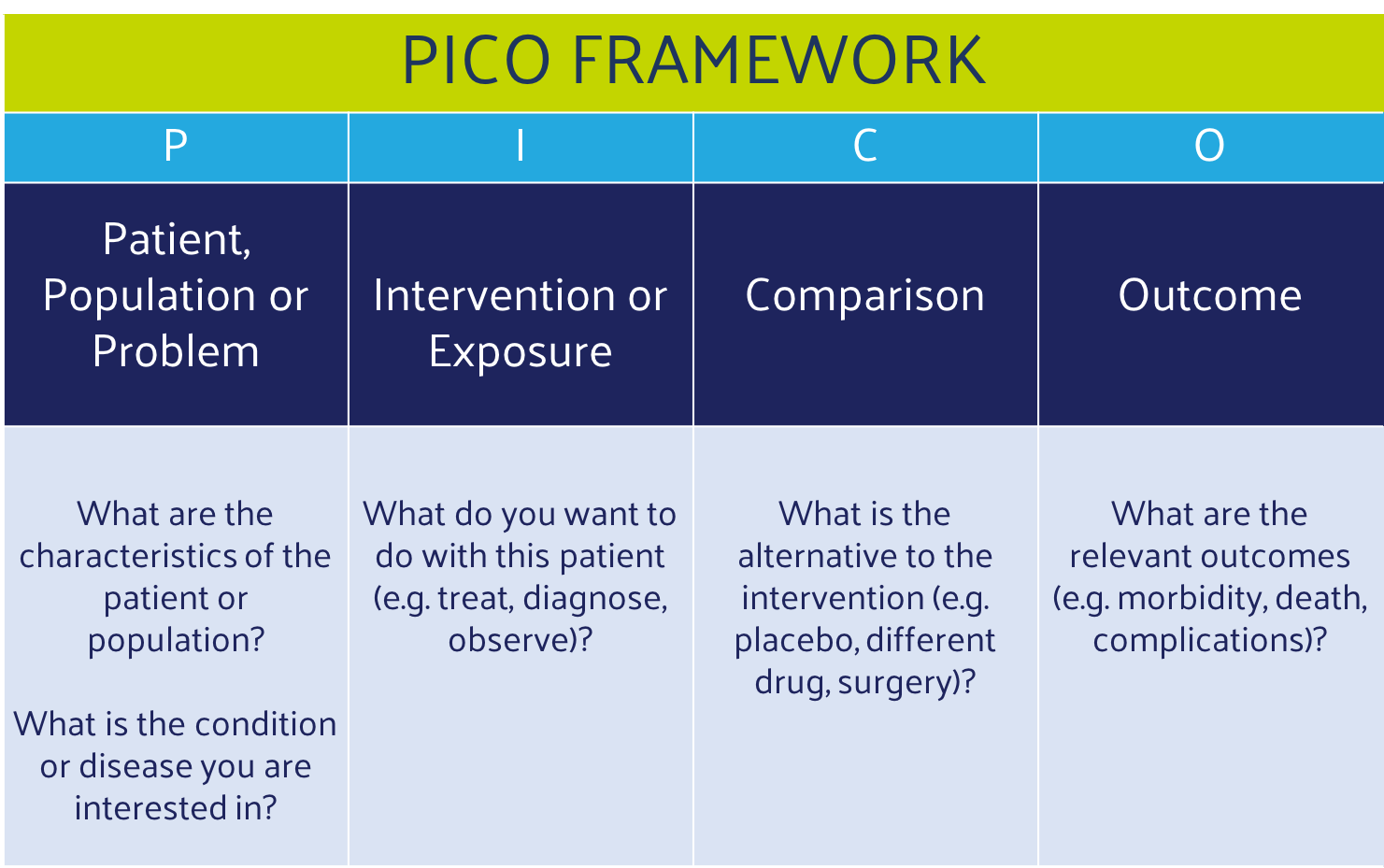
PICO is a standard approach to crafting research questions for evidence-based medicine. PICO questions are highly effective when used to:
- Create a clear question
- Identify the information needed to answer that question
- Translate the question into searchable terms
- Develop and refine the search approach
- Screen, summarize and appraise data
Once your question is established, the next step in your literature review is to track down any research that is potentially relevant to answering the question. This can be a daunting task, which brings us to tip #2:
Enlist an information specialist for best results
Information specialists can be a huge asset to your review team. They know all the tricks for developing a search strategy that works! These may include things like:
- Synonyms, singular/plural forms, verbal forms, adjectives, different spellings
- Classification terms used by databases
- Boolean search operators e.g.: ‘AND’, ‘OR’, ‘NOT’
- Using a truncation symbol to look for all words starting with a particular combination of letters (use ‘$’ or ‘*’ depending on the database)
- Coming up with a search strategy that effectively hones in on the relevant studies while minimizing the noise from irrelevant ones is definitely a job for an expert.
With regulatory bodies calling for continuous monitoring and assessment of safety data, particularly for compliance with MEDDEV 2.7/1 rev.4 in the EU, literature reviews are increasingly scrutinized for thoroughness, standardized processes, and data integrity. Follow these two tips, and your literature review will be off to a great start!
A little More about PICO
PICO Research Question: A Key to MDR-Ready Literature Reviews
Literature reviews are an essential part of evidence-based practice (EBP). They’re the gold standard in research in critical fields, so practitioners must take care in ensuring their success. The best way to do that is to start with a question based on the PICO framework for your medical device regulation (MDR) literature review.
PICO is a framework for literature review that helps focus a research’s objective and search strategy. PICO serves as a solid foundation for PICO medicine initiatives.
What is the difference between a PICO question and a research question?
A research question is a general query posed to answer a question related to a chosen topic. A PICO question is a type of research question that is more specific compared to other questions.
The PICO model is designed to focus evidence-based research on relevant components: patients, population, or problem (P), intervention (I), comparison (C), and outcome (O). The question identifies key terms for a study, informing its search strategy and guiding its process. Here are some examples PICO questions.
Do qualitative studies use PICO?
PICO is often used in quantitative studies, but it can also be adapted for qualitative reviews, which are becoming increasingly common in population health, education research, and healthcare service.
What are the disadvantages of the PICO framework?
While the PICO framework element acts as the standard for EBP research questions, PICO has some limitations. For example, while PICO can be used for qualitative research, it may not be suitable or applicable to all research questions.
What are the best types of study designs utilized for a PICO question?
You can use multiple study designs with a good PICO question. Here are some PICO examples:
Systematic review: This is a study that involves a detailed plan to identify, appraise, and summarize relevant studies to answer a well-defined question.
Meta-analysis: This is a statistical technique that summarizes the conclusions of multiple studies in a single weighted estimate, and it can be done within a systematic review.
Randomized control trial: This would be a trial that assigns participants in experimental and control groups to assess the relative effects of medical intervention.
Controlled clinical trial: This trial is similar to a randomized controlled trial, except participants are assigned into groups (which could make it susceptible to bias).
Cohort study: This study follows a group of people, called a ‘cohort,’ to see differences in how events happen to people. It can determine whether exposure to a risk factor can cause a specific event.
Case control study: This is a study of two groups of people–one that has experienced a certain event and another that hasn’t–done to see the effects of exposure to suspected agents.
What is the alternative to the PICO question?
You can use another question framework as an alternative to PICO–there are at least twenty-five other question frameworks. Some common question frameworks include:
PEO Questions: For qualitative research topics
SPIDER Questions: For qualitative or mixed methods research topics focused on samples (rather than populations)
SPICE Questions: For qualitative research topics assessing the outcomes of a service, project, or intervention
ECLIPSE Questions: For qualitative research topics looking at the results of a policy or service








