*This post was updated in April 2020 to reflect the postponement of the EU MDR deadline.
Although the EU MDR deadline has been officially pushed back to May 2021, device manufacturers are still facing a dramatically increased regulatory workload. When this is multiplied by time pressures and a lack of expert resources or bandwidth, it equals an amplified risk of regulatory non-compliance.
When it comes to literature reviews in the age of MDR, are you compliant? How do you know if you aren’t?
What Notified Bodies Will Be Looking For
Before you can produce compliant literature reviews you first need to understand what they entail. There are many things notified bodies will be looking for in your clinical evaluation report (CER) literature review, including:
- Search strategy
- Databases used
- Search output
- Included/excluded reference lists (with reasons for inclusions or exclusions)
- Relevant study summaries
- Coverage (did you find all the relevant papers?)
- Appraisal and weighing of relevant studies
- Transparent and reproducible processes
- CVs of qualified reviewers
Common Points of Failure
Now that you have a sense of the things that will be under scrutiny by the notified bodies, let’s talk about some of the most common issues with CER literature review processes:
- Incomplete Search Coverage: This is the number one mistake made in the preparation of literature reviews. Because of the work involved in screening large volumes of literature, some groups intentionally narrow their search to limit the amount of screening needed. A search that is too narrow or doesn’t include enough sources runs the risk of missing a key piece of literature and invalidating your results.
- Incomplete Audit Trail: Notified bodies want to see your path. It is essential to show your work and remain as transparent as possible.
- Ad Hoc Processes: The process for the review should be standardized and demonstratively repeatable to minimize errors.
- Data Integrity Errors: Manually copying and pasting and free form text input will almost always result in errors, typos, and inconsistencies in your data.
- Efficiency: Literature reviews deal with a huge amount of data. It’s critical that the review process can be reproduced efficiently and scale effectively to fulfill the ongoing requirements of the new regulations.
Literature Review Best Practices
1. Optimize the Reference Screening Processes
Screening your references is the first step, and the success of your review depends heavily on getting this process right. Some of the best practices for optimizing your reference screening process include:
- Dual Screening: Humans make mistakes; it’s important to use multiple reviewers to validate inclusion/exclusion decisions during screening.
- Two-Step Screening: First, screen the title and abstract then screen the full text. Full text takes longer to screen and articles often come at a cost, so it’s more efficient to screen the summary first and then only procure the full text for the references that look promising.
- Capture the Reasons for Exclusion: This is a short step that is often omitted at initial screening. It might seem unnecessary, but will save you significant time in the future. You need to be able to answer the question, “Why did you exclude that paper?”. Also, if your inclusion criteria change in the future, or if you are considering the paper for another review, having your exclusion reasons documented may prevent you from having to screen the same paper again.
- Employ Automation: When reviewing on a massive scale, it’s critical to leverage automation technology to streamline time-consuming tasks like title and abstract screening. Artificial intelligence technology is also available to enhance the accuracy of deduplication and score references by likelihood of inclusion.
2. Use Tools with Audit Trails and Version Control
Notified bodies want to see a transparent process with an easy-to-follow audit trail. Therefore, all entries or changes should be tracked, time-stamped, and attributed to a writer and all the original data should be retained.
Version control and audit logs can also dramatically accelerate the process of tracking down data errors and detecting data entry mistakes.
3. Use Template Forms for Screening Data
Templates allow you to create a prescriptive and standardized review process that makes it easier to manage multiple reviews, introduce/train new team members and ensure consistent reporting formats.
The best way to ensure your templates are MDR-compliant is to develop a set of standardized forms that mirror the requirements. You can then use the templates across your portfolio of reviews, always in the same format for increased efficiency and guaranteed compliance.
4. Develop an Evergreen Review Process
Regulatory literature reviews are not a one-time effort; they must be updated for resubmission on a periodic basis. For this reason, it is critical to develop a process that allows updates to occur with minimal rework. Ideally, your process should allow you to easily incorporate newly available evidence into a living review, allowing you to produce updates by only reviewing what has changed since the last update.
While living reviews may require protocol updates from time-to-time, they should remain mostly the same.
For example:
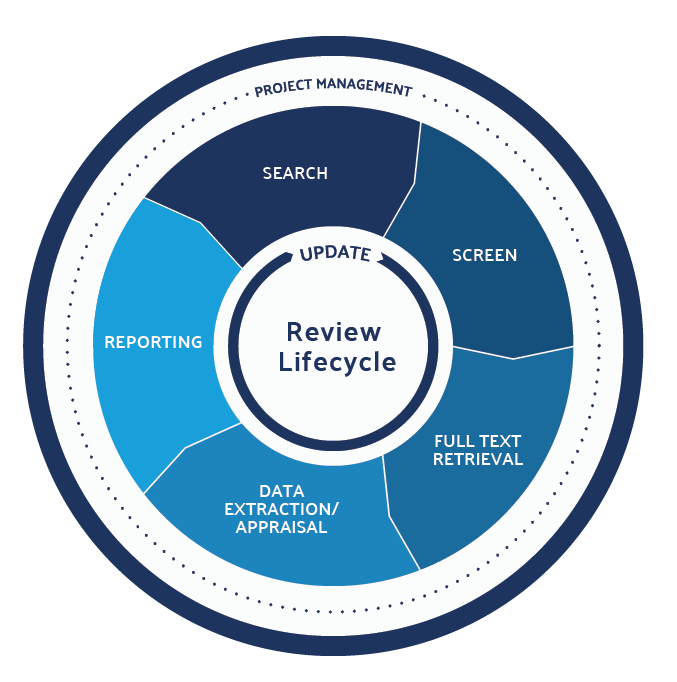
5. Aggregate Coded References for Re-use
In conducting a literature review, you are essentially producing structured datasets extracted from your references. This data is valuable and should be stored for potential reuse so that you never need to use people to extract that data again.
By collecting aggregate data in a data repository, you are building a proprietary knowledge base of structured content. This is an information asset. Reference management, homegrown databases and share repository software can help to curate your coded reference repository.
When contemplating data reuse, it’s important to develop a standardized coding lexicon upfront and, if possible, designate a knowledge manager to oversee repository additions.
Are you ready for the crunch?
The new EU MDR has big implications when it comes to literature review. Device manufacturers need to pay close attention to literature review methodology and scalability.
By considering these best practices, you will be well on your way to creating a streamlined, repeatable literature review process that fully addresses your regulatory obligations.








